Organic Chemistry
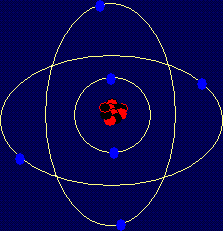
Carbon is element number 6 in the Periodic Table and has 4 electrons in its outer shell, as you can see from this diagram. Because there are 4 electrons in the outer shell, carbon always forms compounds with 4 covalent bonds in them. These are normally drawn using 4 straight lines. Some examples of carbon compounds are given below. I have represented the atoms by their chemical symbols (C for carbon, H for hydrogen etc.), which is very common when drawing organic molecules.
A word about notationOrganic compounds are generally represented in the way that you see above, with the symbols for each element joined by lines. The structure of the molecules is, of course, three-dimensional. The four bonds that join a carbon atom to other atoms in a compound are made up from electron sharing, and electrons, being negative, naturally repel each other so that the bonds are as far away from each other as possible. 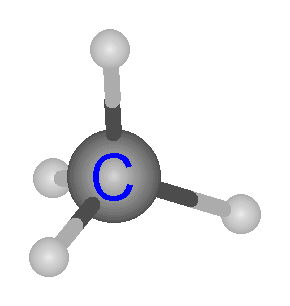
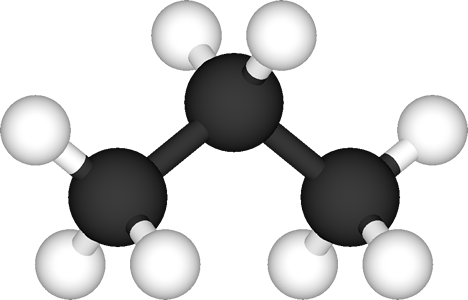
This means that the bonds form a tetrahedral structure (similar to a pyramid, but with a triangular base). This is best shown with methane. The single carbon atom has four hydrogen atoms bonded to it, with three forming a triangular "base" and one more at the apex of the tetrahedron. Compare this 3-D diagram with the "flattened" representation of methane at the top of the page. More complicated carbon compounds have a similar structure. Compare the realistic representation of propane (below left) with the more usual flat representation (below right)
Whereas this is the correct arrangement of atoms in real life, it soon becomes impractical to show complex molecules like this, so we generally stick to the flat notation. That is what you will see in this tutorial. |
 This is the study of one particular element - carbon. It's often called Carbon Chemistry. Carbon forms an almost infinite number of compounds. All life forms are based on a huge number of carbon compounds. Any molecule that contains only carbon and hydrogen atoms is also called a hydrocarbon
This is the study of one particular element - carbon. It's often called Carbon Chemistry. Carbon forms an almost infinite number of compounds. All life forms are based on a huge number of carbon compounds. Any molecule that contains only carbon and hydrogen atoms is also called a hydrocarbon
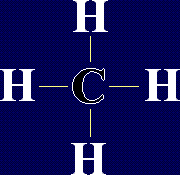 This is methane, the first of a family of organic compounds called alkanes. Its formula is
This is methane, the first of a family of organic compounds called alkanes. Its formula is  This is Carbon Dioxide,
This is Carbon Dioxide, 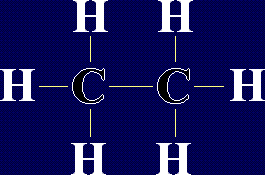 This is the next alkane in the series, ethane, formula
This is the next alkane in the series, ethane, formula 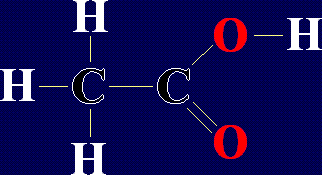 This molecule is a type of organic compound called an Organic Acid. In fact, it is
This molecule is a type of organic compound called an Organic Acid. In fact, it is