Acids and AlkalisWhat are acids and alkalis?
We almost always write a hydrogen ion as H+. However, as it hides inside a water molecule (technically becoming a hydroxonium ion, although we don't usually call it that), we should really write it as H3O+. Either way, it has a single positive charge on it. Strong and weak acidsAcids like sulphuric acid, nitric acid and hydrochloric acid are called strong acids. This means that they dissociate almost completely when dissolved in water, i.e. almost all of the acid is in the form of hydrogen ions and the negative ions associated with the acid (sulphate, chloride or whatever). However, weak acids such as ethanoic acid only dissociate partially. The covalent molecules break up into hydrogen ions and ethanoate ions, but these ions start to recombine to form covalent ethanoic acid. In this way, an equilibrium is set up in which the rate at which covalent ethanoic acid is breaking up to form ions is the same as the rate at which the ions are recombining to form the covalent molecules. We can write this equilibrium as a reversible equation with a double arrow to show that it proceeds in both directions at once:
CH3COOH (aq)
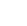 CH3COO- (aq) + H+ (aq) CH3COO- (aq) + H+ (aq)
A reaction similar to this occurs when any acid is dissolved in water, of course, but for strong acids the result is almost entirely dissociated ions (the right side of the equation). Reactions of AcidsThere are four common reactions that acids have. Anything that reacts with an acid is called a BASE. Any base that dissolves in water is called an ALKALI. Here are the four common things that you learn about at GCSE that react with acids. Click on each one to learn something about it. The pH ScaleThe strength of an acid or an alkali can be measured by the concentration of hydrogen ions in it. The more hydrogen ions it contains, the more concentrated it is. For convenience, this concentration is turned into a number called the pH of the acid (standing for 'hydrogen potential'). As the pH of a liquid goes down, it becomes more acidic (less alkaline). As the pH of a liquid goes up, it becomes more alkaline (less acidic). The pH scale goes from 0 (strongly acidic) to 14 (strongly alkaline) with pure water (neutral - neither acidic nor alkaline) at 7 in the middle. The concentration of any solution is represented as moles per litre, i.e. how many moles of the chemical are present in 1 litre (1000 cm3) of water. (For a fuller explanation of moles, see the section on Moles and the Avagadro Constant). This concentration is then written as a power of 10. For instance, pure water consists almost entirely of covalent molecules, although a tiny proportion of its molecules do split up to form hydrogen ions and hydroxyl ions. This means that the concentration of hydrogen ions is low, at 0.0000001 moles of hydrogen ions per litre, or 10-7 moles per litre. A concentrated acid would have a much higher concentration of hydrogen ions, for instance, 0.01 moles of hydrogen ions per litre, which could be written as 10-2, and a strong alkali would have a lower concentration of hydrogen ions than even pure water, for instance, 0.0000000001 moles of hydrogen ions per litre, which could be written as 10-10. Having written the concentration as a power of 10, we simply remove the base number 10 (we call this "taking the logarithm") together with the minus sign, to give the pH value, so a concentration of 10-7 moles per litre of hydrogen ions becomes a pH of 7 (not -7), which is the pH of water (or a neutral solution), and a concentration of 10-2 moles per litre becomes a pH of 2, and a concentration of 10-10 becomes a pH value of 10. IndicatorsThere is a device called a pH meter which you can dip into a liquid and which will measure its pH. However, it is easier to use an indicator. An indicator is a chemical that changes colour. Indicators are liquids, but they can be soaked into a type of paper similar to blotting-paper to form strips of indicator paper. Just dip the indicator paper into the unknown liquid and it will change colour to show the pH. The simplest indicator is Litmus (from the Litmus plant). It goes red in acids, blue in alkalis.
Universal indicator is a better indicator than litmus because it can show a greater range of colours. The chart below shows the approximate colour that universal indicator goes when put into liquids of different pH values.
Universal indicator is available as a liquid or soaked into absorbing paper. It is often used to work out the pH of soil samples to see which plants can be grown in a certain patch of ground. There are other indicators, for example, methyl orange (which changes colour from colourless to orange at pH of about 3). They all have different pH values where they change colour, and each has its own uses. |
 Back to the menu
Back to the menu