The Periodic Table
What is the Periodic Table?It is a list of all the chemical elements which are known. Most of these (the ones up to and including Uranium) occur naturally, but some only exist because they have been created in the laboratory. It is called the Periodic Table because the horizontal rows into which the elements have been arranged are called periods. Why are the elements arranged in that peculiar pattern?Good question. Why not simply arrange them in a rectangular block? It would certainly be easier to read. The reason is that the elements have been arranged to reflect chemical patterns, especially down the vertical columns. For instance, consider the elements on the left side of the table. If you ignore Hydrogen (H) at the top (which tends to be a law unto itself), they are Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs) and Francium (Fr). These elements are metals, and they all show the same chemical behaviour to a greater or lesser extent. For instance, they all react with water (less so in the case of Lithium, more so as you go down the group, until you get to Caesium which reacts explosively with water). They all form alkaline oxides, ions with a single positive charge etc. This is why they have been arranged above one another in the table. These vertical arrangements are called groups, and they have different numbers of elements in them. That is why they are arranged the way they are. Why are some of the elements split off into their own little group at the bottom?Actually, that small separate rectangle of elements at the bottom is two periods. The top row is called the Lanthanides, as the element on the left is Lanthanum (La). The bottom row is called the Actinides, as the element on the left is Actinium (Ac). The elements should really be slotted in where that gap is just after the second group. The Lanthanides should go in just after Barium (Ba) and the Actinides should go in just after Radium (Ra). The connecting lines show where the two periods should slot in: 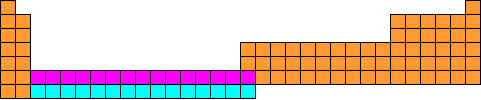 How we should really show the Periodic Table The reason that we don't write the table like this is because it would make it too wide to be easily readable. Instead, we "chop the legs off the corpse so it will fit in the coffin" and write the two periods beneath: 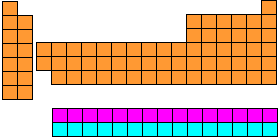 This is how we usually show it. Who first arranged the elements like this?The first chemist to succeed where others had failed in arranging the elements in some sensible pattern was a Russian called Mendeleyev (after whom the element Mendelevium is named). He was a friend of the Russian composer Alexander Borodin (who was also a chemist - he taught chemistry at St. Petersburg University). It was a long hard struggle, made harder by the fact that in the 1860s, when he tackled the problem, only a fraction of the known elements had been discovered. He wrote the names of the 60 or so elements that were known in his time on a set of playing cards and spent months arranging them and rearranging them looking for patterns, not only down the columns but along the rows as well. Eventually he hit upon the solution. He claimed that the answer came to him in a dream, though how much credence we should give to that is debatable. Anyway, he arranged the elements in the pattern that we now call the periodic table, even though it had a great many gaps in it! However, the fact that there were large numbers of gaps in Mendeleyev's table suggests that there were as yet unknown elements that should go in those gaps. He was overjoyed when the elements Scandium, Germanium and Gallium were discovered in the 1870s and 1880s as they fitted perfectly where he predicted in gaps in the table, and they had the properties that he predicted they should have. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 Go back
Go back