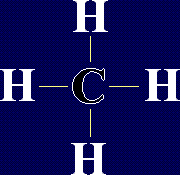
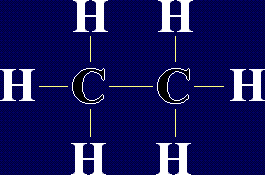
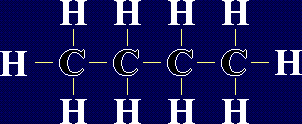
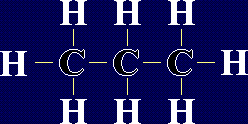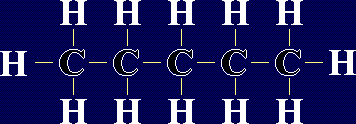Organic Chemistry - AlkanesAlkanes are a family of chemicals which are compounds of carbon and hydrogen only. They occur naturally - in fact, crude oil is a mixture of a large number of alkanes (and a few other chemicals). The general formula for any alkane is CnH2n+2, where n is any whole number. This means that the number of hydrogen atoms in an alkane is always 2 more than double the number of carbon atoms. The first few members of the alkanes are shown below:
What do alkanes do?They burn, which is why they make superb fuels. All small alkane molecules will burn in oxygen to form carbon dioxide and steam (which condenses to form water): Alkane + Oxygen → Carbon dioxide + water
Larger alkane molecules, with more than about 15 carbon atoms in them, burn less readily. Alkanes can also burn incompletely. If there is not enough oxygen, then the reaction produces carbon monoxide instead of carbon dioxide:
This is the main reason that methane-burning boilers must be serviced regularly to ensure that they burn the methane "cleanly". Carbon monoxide is highly toxic, and an unserviced boiler can produce this "silent killer". Fair enough, they burn. What else do they do?When mixed with halogens (group 7), alkanes undergo a type of reaction called substitution. As you may have guessed, this involves a pair of atoms swapping their positions. Halogen elements exist in the form of diatomic molecules, i.e. molelcues containing two atoms. A molecule of chlorine, for example, contains two chlorine atoms, Cl2. What happens is as follows: One of the hydrogens on the alkane detaches, as does one of the halogen atoms from the molecule. The loose halogen atom attaches to the alkane where the hydrogen was, forming a haloalkane, and the loose hydrogen attaches to the remaining halogen atom, forming a hydogen halide. | |||||||||||||||||||||||||||||||||