Hydroxonium IonsHydrogen atoms consist of one proton orbitted by one electron. If they were simply to lose their electron, they would be a lone proton, which isn't stable. This is why hydrogen atoms can only become hydrogen ions in situations where they can "hide" inside orbitals of other molecules. 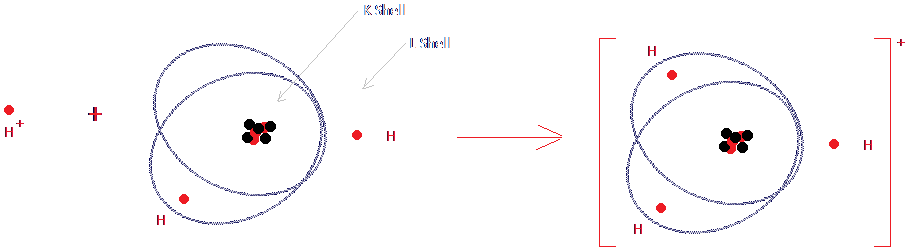 If you have looked at the tutorial on Atomic Structure, you will know that oxygen atoms have two electron shells - the K shell and the L shell. There are two electrons in the K shell, forming a spherical orbit. However, the L shell does not consist of a simple spherical shell. The first two electrons in the L shell are in a spherical orbit, and the other electrons are in pairs in parabolic (oval-shaped) orbitals. Take a look at the diagram above. The L shell is that complicated shape made up of a sphere and those overlapping parabolic orbits. In a water molecule, there are already two hydrogen atoms (protons) hiding in two of those orbitals - those orbitals are the covalent bonds you have been hearing about - but one of the parabolic orbitals is empty. The hydrogen ion hides in that empty orbital, as shown in the diagram. Now the water molecule has in total one more proton than electron, and the whole thing has the single positive charge thanks to the hydrogen ion that has hidden within it. Although we should really write the hydroxonium ion as H3O+, it's generally too complicated, so we just write it as a simply hydrogen ion, H+. However this does explain why acids only dissociate into ions in the presence of water molecules.  Go back Go back |