
Atomic StructureIntroductionAll normal matter consists of atoms, which are often joined together to form molecules. All atoms are made of three types of particle:
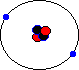
The protons and neutrons contain 99% of the mass of the atom. They form the nucleus at the centre of the atom. The electrons orbit the nucleus like planets orbitting a sun. The nucleus is where most of the mass of the atom is concentrated. The nucleus takes up an astonishingly small amount of space in the atom. The image shown is not to scale. In fact, if an atom were enlarged to the size of Wembley Stadium, the nucleus would still only be the size of a pea! This means all atoms, and all matter made from them, is 99% empty space. You may have met the following law about electrostatic forces when you studied physics:
Opposite charges (positive and negative) attract. Like charges (positive and positive, or negative and negative) repel.
This means that the negative electrons orbitting around the positive nucleus are attracted to it, and it is this attraction which keeps them in orbit, and stops them from flying off into the void. Their orbital speed (which is high!) and various quantum effects (don't ask!) stop them falling in towards the nucleus and crashing into it.
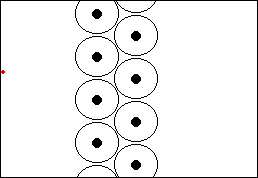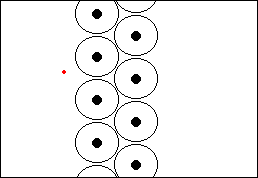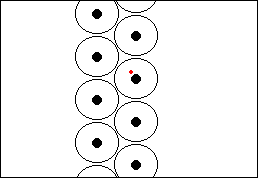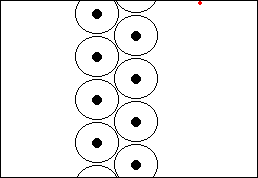
Rutherford's ExperimentHow do we know all this? Well, due to experiments performed by scientists such as Ernest Rutherford at the end of the nineteenth and beginning of the twentieth century. In 1910, Rutherford did an experiment to determine what atoms were like. He fired a beam of alpha particles (which had recently been discovered) at a thin gold foil, tracking them using a detector. To his surprise he discovered that most of the particles went straight through the foil, but some were deflected at an angle and some even bounced straight back! This showed that most of the particles passed through empty space but that some were being deflected by a small point charge. Alpha particles were known to be positive so they must be bouncing off the positive nucleus.
Atomic Number and Relative Atomic Mass
The example that you see above is the symbol for Gold (Au). It is 197 times as heavy as a hydrogen atom, i.e. its Relative Atomic Mass (R.A.M.) is 197. The bottom number (the Atomic Number) tells you how many protons there are in the atom. It also marks the atom's position in the Periodic Table, since they are arranged in the order of their proton count. You can work out how many neutrons there are in the atom by subtracting the bottom number from the top. The example shows us that gold is element number 79 in the table, and therefore each atom of gold has 79 protons in it. The number of neutrons in a gold atom is 197 - 79 = 118.
Would you like a question about this? If so, click on the icon on the right. If you look at the periodic table, you will find that the elements all have relative atomic masses which are decimal values. How can you have a fraction of a neutron? Well, clearly atoms don't contain parts of neutrons. The effect occurs because each element consists of a number of isotopes. In practice, most relative atomic masses are so close to whole numbers that we tend to round them. For instance, the relative atomic mass of Chromium is 51.996, so we tend to use the value 52 as the relative atomic mass. IsotopesThe example that is always used to demonstrate isotopes is chlorine, and I am no exception, so I shall use that example. If we simplify reality a little, we can say that there are two types of chlorine atom - one has 17 protons and 18 neutrons (and so has a relative atomic mass of 35, and is termed Chlorine-35) and the other has 17 protons and 20 neutrons (and so has a relative atomic mass of 37, and is termed Chlorine-37). Both sorts of chlorine atoms have the same number of protons (17). After all, that is what makes the atom a chlorine atom! If the atom didn't have 17 protons, then it would be an atom of some other element. Both sorts of atoms behave in exactly the same way chemically, and there is no chemical way to separate them. Atoms with the same number of protons but different numbers of neutrons are called isotopes. Chlorine-35 and Chlorine-37 are both isotopes of chlorine. All elements have different isotopes. Chlorine consists of roughly 75% Chlorine-35 and roughly 25% Chlorine-37. All the atoms are mixed together randomly, and we can't separate them. So what should we call the relative atomic mass of chlorine? We take an average of the two figures which is weighted towards the figure 35 to take into account the fact that Chlorine-35 is three times more common than Chlorine-37. The relative atomic mass of chlorine is usually quoted as 35.5.  Of course, there are really more than two isotopes of chlorine, which is why the exact figure for chlorine is not 35.5, but this is close enough to the real truth. More IsotopesTwo common ones are Uranium-235 (with 92 protons and 143 neutrons) and Uranium-238 (with 92 protons and 146 neutrons), which are both isotopes of uranium. There are three isotopes of hydrogen: plain hydrogen (1 proton and no neutrons), the very rare deuterium (1 proton and 1 neutron) and the even rarer tritium (1 proton and 2 neutrons). These are all types of hydrogen and behave in exactly the same way.
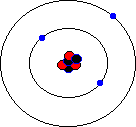
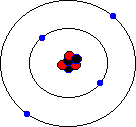
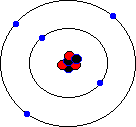
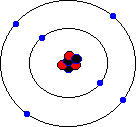
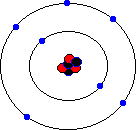
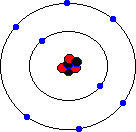
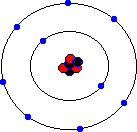
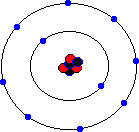
You may hear about an isotope of carbon called Carbon-14. Unlike the great majority of carbon atoms (called Carbon-12 as their nuclei have 6 protons and 6 neutrons), Carbon-14 atoms have 8 neutrons. This rare isotope of carbon is formed in the upper atmosphere when cosmic rays strike nitrogen atoms, and is taken in by plants through photosynthesis (plants absorb carbon dioxide gas through their leaves). The plants are eaten by animals, and so carbon-14 atoms are found in all living things. Carbon-14 atoms are radioactive and decay with a very precise and long half life. This means that the age of any fossil or archaelogical exhibit that used to be part of a living thing (e.g. timbers from a Viking longship, cloth fibres, or leather from an ice-age belt) can be dated accurately. This process is known as radio-carbon dating. Its most famous use was to prove that the Turin shroud, once thought to be the cloth shroud in which Christ was buried, was in fact a fourteenth century fake! Electron shellsYou might think that electrons would orbit the atom willy-nilly without any particular order. In fact, they are found at certain distances from the atom, called electron shells or electron orbitals. At G.C.S.E. you need to know these basic shells, but at A-Level, you will learn a slightly more complex version of the model. You can think of electron shells as being like parking places. They gradually "fill up" as you go from smaller atoms to larger ones, with the innermost shell filling up first, then the second shell out, etc. Since electrons are negatively charged, and protons are positively charged (same charge value but positive instead of negative), then for an atom to be neutral (no overall charge), the number of electrons must equal the number of protons. This means that an element with, say, 28 protons (that's iron, incidentally) would have to have 28 electrons as well. However, one thing that you do learn about electrons, even at G.C.S.E., is that they are sometimes capable of leaving an atom and travelling to another one. This means that atoms may have more electrons than protons (in which case we call them negatively charged ions rather than atoms) or fewer electrons than protons (positively charged ions). In the sections that follow we will only consider atoms that are neutral, i.e. we will assume that the numbers of protons and electrons match. The K-shellWhen I was young, the innermost shell was called the K-shell (I think they call it simply "shell 1" when you reach A-Level). It can hold, at most, two electrons before it becomes full. The first element in the periodic table, hydrogen, has one electron in its K-shell, and one "gap" in the shell. The next element, helium, has two electrons in its K-shell, which is therefore full:
The L-shellThe next shell is referred to as the L-shell, and the electrons in it orbit further away from the nucleus than those in the K-shell. The L-shell (shell 2) can hold a maximum of 8 electrons before it fills up. Here are the next 8 elements. You will notice that each atom has one more electron than the last and that they fill up the second shell:
The M-shellNow that the L-shell is full, we have to start filling the next shell out, the M-shell or Shell 3, which can again a maximum of 8 electrons. Sodium is element number 11, so a neutral sodium atom has a total of 11 electrons (2 in the first shell, 8 in the next shell out, and 1 in the third shell). Magnesium has 12 electrons (the same as sodium except that it has 2 in the third shell). Aluminium has 13 electrons, with 3 in the third shell etc. This goes all the way up to element 18, which is Argon (2 electrons in the innermost shell, followed by 8 electrons, and then 8 electrons in the third shell). Argon represents the point where the third shell is full - well, that's not entirely true. The third shell actually has some extra orbitals, called "d" orbitals, which can hold an additional 10 electrons. However, these are higher energy orbitals, which mean that they don't fill up quite so easily, and the third shell can be considered as "full" when it holds 8 electrons. It certainly behaves as if it is full when it holds 8 electrons.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Positively charged protons
Positively charged protons Uncharged neutrons
Uncharged neutrons Negatively charged electrons
Negatively charged electrons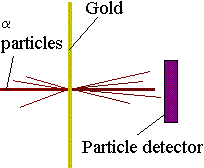
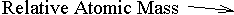
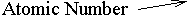




 Go back
Go back