ElectrolysisThis is the study of electric currents passing through various liquids and the breakdown of those liquids as a result of the electricity passing through them. When a liquid breaks down chemically due to electrolysis, we call that liquid an electrolyte, and we say that we are electrolysing the liquid. We can't electrolyse every single liquid. For instance, petrol will not conduct electricity, and neither will liquid sulphur. Electricity involves moving electrical charges, so any liquid in which charged particles are free to move will conduct electricity. In practice, this means one of two types of liquid:
The current passes into and out of the electrolyte through two connections called electrodes. At the negative electrode, called the cathode, electrons are passed into the electrolyte and are taken up by the ions in it. At the positive electrode, called the anode, the ions in the electrolyte give up some electrons which then flow away along the wires. The electrodes can be made of any material that can conduct electricity. In practice, this means either a metal (such as zinc or copper) or graphite which is a special form of carbon, in which the atoms are arranged so that some of the electrons are free to pass between them. As a result, graphite is a form of carbon which conducts electricity (it's the "lead" that you find in pencils). What ions are present?Water is generally covalent, but a small proportion of the covalent water molecules break up into hydrogen ions and hydroxide ions:
H2O (l)
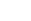 H+(aq) + OH-(aq) H+(aq) + OH-(aq)
Note that the reaction is reversible. At the same time as water molecules are breaking up into ions, hydrogen ions and hydroxide ions are recombining to form water molecules. All this means that water is actually a relatively poor conductor of electricity! However, it does conduct well enough to be dangerous, so I would recommend against touching any electrical equipment when you have wet hands or taking anything electrical into the bathroom. (In fact, a simple hydrogen ion, H+ would be a lone proton, which would be unstable, so they can only exist if they hide inside water molecules, to produce a water molecule with an extra proton hiding in it. This is called a hydroxonium ion, H3O+. However, the carrier water molecule takes no part in any chemical reactions, so we can ignore it, and just treat the hydrogen ion as if it were simply H+). The upshot of all this is that any solution that conducts electricity (i.e. when a salt or acid is dissolved in water) will contain hydrogen ions and hydroxide ions, in addition to whatever ions the salt or acid provides. Apart from that it is fairly obvious: sodium chloride, whether it is dissolved or molten, will contain sodium ions and chloride ions; magnesium carbonate contains magnesium ions and carbonate ions etc. Matters are a lot simpler when an ionic substance is molten ("fused"). In this case, there are only two types of ion present, one positive, one negative. For instance, when sodium chloride is heated strongly enough, it eventually melts, and the positive sodium ions and negative chloride ions are free to move around each other. What happens at the Cathode?The cathode is the point where the electrons travelling round the circuit enter the electrolyte. Think of it as a free supply of electrons to the chemicals in the electrolyte. Of course, the cathode is strongly negatively charged, and attracts the positive ions in the electrolyte, which flow freely towards it (they can move around, remember!) When the ions reach the cathode, they pick up electrons and become neutral atoms. This means that the chemical element that they represent (hydrogen, potassium, copper or whatever) comes out of the electrolyte as a simple element (i.e. not chemically bonded to other atoms). The basic reaction is as follows: X+ + e- → X
In this case, I have used X to represent some general element rather than any one in particular. Also, note the special symbol e-. This represents a lone electron, which in this case, neutralises the positive ion X+ to produce the electrically neutral element X. The symbol has a negative sign associated with it to show that the electron that it represents has a single negative charge. In that equation above, I haven't included any states of matter (remember (l) for liquid, (aq) for dissolved in water etc.). This is because we don't know whether those positive ions are in a solution (which would need (aq)) or a molten compound (which would need (l)). Similarly, we don't know if the element produced is a gas (in the case of hydrogen), a solid (in the case of a metal like copper) or even a liquid (in the case of sodium metal, which is produced by electrolysis at a very high temperature - far above its melting point!) Remember, I'm trying to keep things general! Here are some real examples. Firstly, copper ions in a solution of copper sulphate precipitate out on the cathode to produce a thin coating of metallic copper: Cu2+ (aq) + 2 e- → Cu (s)
Mercury ions are produced at the cathode when a molten mercury salt such as mercury nitrate is electrolysed to produce liquid mercury: Hg+ (l) + e- → Hg (l)
Finally, hydrogen ions in a solution of hydrochloric acid turn into gaseous hydrogen at the cathode:
H+ (aq) + e- → H (aq)
2 H (aq) → H2 (g) Ironically, if you look at those three examples, only the mercury one actually matches the general reaction I gave above perfectly. What's happening with the other two? Consider the copper. Copper ions (almost) always have two positive charges, so each ion requires two electrons to neutralise it. Each electron removes one of the positive charges on the copper ion, so it is necessary to double up on the number of electrons. The hydrogen ion is simpler - it only has one positive charge, so only requires one electron to neutralise it. However, when the individual hydrogen atoms are produced, they are not stable on their own. Hydrogen is a diatomic gas, which means that the atoms join up into pairs to form a (relatively) stable hydrogen molecule, H2. This is represented by the second line of the hydrogen example. You can see that all three examples given are actually variations on the general reaction I gave above.
An example - copper sulphate solutionConsider the example of a solution of copper sulphate being electrolysed. There are two types of positive ion present: Cu++ (from the copper sulphate) and H+ (from the water). Which one comes out at the cathode? Take a look at the simplified version of the reactivity series that you see above. Hydrogen is further up than copper, so the copper ions come out of solution as copper metal and the hydrogen ions stay where they are in solution. Copper sulphate solution is a beautiful sky-blue colour, due entirely to the presence of copper ions. As the solution is electrolysed, the copper ions are gradually removed from solution, so the blue colour slowly fades and disappears. At the same time, the cathode becomes a shiny copper-pink colour as a thin coating of copper metal is deposited on it (and sticks to it!) Also, as the number of copper ions in solution drops, the hydrogen ions become the majority, and eventually - when all the copper has been removed - the only positive ions in solution are hydrogen ions. This means that the solution has changed from being one of copper sulphate (CuSO4) to one of sulphuric acid (H2SO4). As the blue colour fades, you find the electrolyte turns blue litmus paper red! There comes a point when all the copper ions have been removed from solution and the only positive ions left are hydrogen ions. You can tell when this happens, as bubbles of hydrogen start to appear on the copper-pink cathode. What happens at the Anode?Ah, this depends on what the anode is made of, and is a lot more complicated than what happens at the cathode. Here I have summed it up.
In both these cases, you will notice that there is a + e- on the right-hand side. This represents the electrons being stripped off the atoms or ions and pumped up the anode. I think the best thing to do is to give examples. Electrolysis of Silver Nitrate solution with a copper anode
Positive ions present: Ag+, H+ At the cathode, the silver ions will precipitate as metallic silver on the cathode. You will notice that I didn't say what the cathode was made of - I didn't need to, as it doesn't affect what happens at all. Hydrogen won't precipitate out as hydrogen gas, as silver is below it in the reactivity series. Ag+ (aq) + e- → Ag (s)
At the anode, the copper metal gives up its electrons and becomes copper ions in solution. The copper anode gradually crumbles away. Cu (s) → Cu2+ (aq) + 2 e-
In addition to the copper anode getting smaller and smaller, you will notice that the colourless electrolyte gradually turns sky-blue. This is because the silver ions in solution are gradually replaced by copper ions, so the electrolyte changes from silver nitrate to copper nitrate. Electrolysis of Silver Nitrate solution with a graphite anodeThis is essentially the same as the example above - same ions present in solution - with one exception and that is what happens at the anode. It is not possible for the anode to produce positive ions, as carbon doesn't do that whatever form it's in! Instead, the hydroxide ions break up in a rather complicated reaction which produces oxygen bubbles and water molecules: 2 OH- (aq) → H2O (l) + O (aq) + 2 e-
2 O (aq) → O2 (g)
I have given the reaction in two steps. The first step shows a single oxygen atom being produced, and the second step shows the loose oxygen atoms pairing up to form diatomic molecules of oxygen gas. Sometimes, textbooks lump these two steps into one reaction: 4 OH- (aq) → 2 H2O (l) + O2 (aq) + 4 e-
|
 The diagram shows electrolysis taking place. The electric current is carried through the wires by electrons that come out of the negative terminal of the battery or power source, pass round the circuit, and re-enter the battery through the positive terminal.
The diagram shows electrolysis taking place. The electric current is carried through the wires by electrons that come out of the negative terminal of the battery or power source, pass round the circuit, and re-enter the battery through the positive terminal.
 Go back
Go back