Balancing Chemical Equations
What is a chemical equation?When a chemical reaction occurs, it can be described by an equation. This shows the chemicals that react (called the reactants) on the left-hand side, and the chemicals that they produce (called the products) on the right-hand side. The chemicals can be represented by their names or by their chemical symbols. Unlike mathematical equations, the two sides are separated by an arrow, that indicates that the reactants form the products and not the other way round. A large number of chemical equations are more complicated than the simple ones you will see in this section. They are reversible, which means that the reactants react together to form the products, but as soon as the products are formed, they start to react together to reform the reactants! Reversible equations proceed in both directions at once, with reactants forming products and products forming reactants simultaneously. Eventually, the system settles down and a balance (an equilibrium) is reached, with the reactants and products present in stable concentrations. This does not mean that the reaction stops, merely that it proceeds in both directions at the same rate, so that the concentrations do not change. Reversible reactions are indicated with a double arrow as shown in the example below: Ethanoic acid + ethanol
 ethyl ethanoate + water ethyl ethanoate + waterThe concept of balancing equationsTake a look at this chemical word equation: Aluminium + Oxygen → Aluminium Oxide
This is the equation for the burning of aluminium in oxygen. If we convert each of the chemical names into the appropriate symbols, we get the following: Al + O2 → Al2O3
Note that oxygen gas is diatomic, which means that the oxygen atoms, like policemen, go around in pairs. A molecule of aluminium oxide consists of two aluminium atoms combined with three oxygen atoms. Actually, technically the word "molecule" is inappropriate in that previous sentence. The formula simply tells us the ratio of aluminium atoms to oxygen atoms in the compound. In the solid state, the atoms form a giant structure called a crystal lattice rather than individual discrete molecules. When balancing chemical equations, people often refer to the number of species on each side to avoid this problem. You can see by looking at it that there is something wrong with this equation. If you count the number of atoms of each type on each side, you will see that there is only one aluminium atom on the left side whereas there are two on the right. There are two oxygen atoms on the left side, as compared to three on the right side. This clearly doesn't match.
Left side:
   Right side:
Right side:
We can balance the equation by mutiplying the different atoms and molecules on each side by different amounts. Firstly, multiply the aluminium atoms on the left side by 2:  Al + O2 → Al2O3
Left side: Al + O2 → Al2O3
Left side:
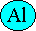 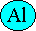
  Right side:
Right side:
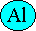 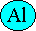   
Now there are the same number of aluminium atoms on each side of the equation. We could also multiply the number of oxygen molecules on each side by one and a half (1.5), which would give three oxygen atoms on the left side (1.5 x 2 = 3) to match the three oxygen atoms on the right side: 2 Al + 1.5 O2 → Al2O3
Left side:
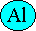 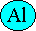
 
 Right side:
Right side:
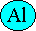 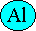   
This is now balanced, but that 1.5 is a horrible thing to have in an equation - how can you have one and a half molecules? We can solve this problem by multiplying everything throughout by 2:  Al + Al +  O2 → O2 →  Al2O3 Al2O3
If you count the number of atoms on each side, you will find that there are four aluminium atoms on each side and six oxygen atoms. Sorted! Another ExampleHere's another equation: Ethane + Oxygen → Carbon Dioxide + Steam
Ethane is a gas similar to methane (town gas or natural gas) which burns in oxygen to give carbon dioxide gas and steam. The steam is simply water in gaseous form and condenses to form water droplets. Here is the chemical equation rewritten with the chemical symbols: C2H6 + O2 → CO2 + H2O
Neither the carbon, nor the oxygen atoms nor the hydrogen atoms match. Let's look at the carbon atoms first. There are two carbon atoms on the left side, but only one on the right, so we need to put a 2 in front of the carbon dioxide molecule to give two carbons on each side: C2H6 + O2 →
 CO2 + 3 H2O CO2 + 3 H2ONow we will look at the hydrogen atoms. There are six hydrogen atoms on the left side and two on the right side, so we treble the number of water molecules on the right side: C2H6 + O2 →
2 CO2 +
 H2O H2ONow there are two carbon atoms on each side, and six hydrogen atoms on each side, but the oxygen atoms don't match. There are 2 of them on the left side and 7 on the right side. This is easily solved by multiplying the oxygen molecule on the left side by 3.5 (as 2 x 3.5 = 7): C2H6 + 3.5 O2 → 2 CO2 + 3 H2O
This gives 2 carbons, 6 hydrogens and 7 oxygens on each side of the equation. The equation is balanced, but rather inelegant since it contains a decimal. Just double all the figures in the equation:  C2H6 + C2H6 +  O2 → O2 →
 CO2 + CO2 +  H2O H2OThe equation has been balanced. You will notice that we left the oxygen atoms until last. This was deliberate, as oxygen was present on one side of the equation as an element (i.e. on the left side of the equation there is oxygen present in an element, not in a compound). Treat standard groups as an itemYou may recognise some standard parts of molecules, ... erm, sorry, species, ... as being a unit. For instance all sulphates contain the group of atoms SO4. These may be doubled (or even trebled) if necessary. Some examples of sulphates are shown below:
You will notice that iron forms two sulphates, depending on its oxidation state. Being a transition metal, it can form different types of ion, Fe2+ and Fe3+ in this case. Lead also forms different ions, but I have just quoted one of its sulphates. To show that the sulphate ion is a single group, it is usually included in brackets when it has to be doubled, so iron (III) sulphate is generally written as Fe2(SO4)3 rather than Fe2S3O12. If you can recognise a standard group, such as suphate, phosphate, nitrate etc., then you should treat it as an indivisible item. It isn't essential to do this, i.e. you can still balance the equation successfully even if you treat each atom individually, but treating groups as special items makes life a little easier. If you are in any doubt, you could temporarily replace the group with a neutral letter such as X (which is not the symbol of a chemical element). Once the equation is balanced, put the group back into place, remembering to insert brackets if necessary. Take the reaction where iron (III) oxide is put in sulphuric acid:
Fe2O3 + H2SO4 → Fe2(SO4)3 + H2O
Let's make life easier by replacing SO4 with X: Fe2O3 + H2X → Fe2X3 + H2O
Now we can balance the equation fairly easily: Fe2O3 + 3 H2X → Fe2X3 + 3 H2O
Replacing X with SO4 gives the final equation: Fe2O3 + 3 H2SO4 → Fe2(SO4)3 + 3 H2O
N.B. The approach of treating standard groups as an item only works if those groups remain unscathed throughout the reaction. If you find that a sulphate species is broken up (perhaps into an oxide of sulphur), then you can't use this approach. This is why balancing chemical equations is so much easier if you have some knowledge of the reactions going on. Balance the elements last!You should leave the elements that appear as elements anywhere in the equation until last. This is because you can balance these elements without affecting any other elements. Here's an example: Under certain circumstances, carbon dioxide can be made to react with hydrogen gas to produce methane and water vapour (which can be electrolysed to produce oxygen and hydrogen - what a way to produce fuel!) CO2 + H2 → CH4 + H2O
Let's do this the wrong way - let's balance the hydrogen first! There are two hydrogen atoms on the left (present in the hydrogen molecule) and six on the right, so we put a 3 in front of the hydrogen molecule on the left: CO2 +
 H2 → CH4 + H2O H2 → CH4 + H2ONow there are six hydrogen atoms on each side. The carbon atoms are balanced, one on each side, so we only have to balance the oxygen atoms. There are two on the left side, and one on the right side. Better put a 2 in front of the water vapour molecule on the right side: CO2 + 3 H2 → CH4 +  H2O H2O
But now the hydrogens are unbalanced again! We either have to increase the number in front of the hydrogen molecule on the left side or add more methane molecules on the right side. Either way, putting a number in front of the water vapour has changed both the hydrogen and the oxygen. The proper way to do it would be to balance the carbons and oxygens and then the hydrogens. Here's the original equation: CO2 + H2 → CH4 + H2O
The carbons are balanced so let's concentrate on the oxygens. There are two on the left and one on the right, which is easily remedied: CO2 + H2 → CH4 +
 H2O H2OThe only element which isn't balanced is hydrogen, which can be balanced without affecting any other elements. There are now eight hydrogen atoms on the right side and only two on the left, so we need to multiply the hydrogen on the left by 4: CO2 +
 H2 → CH4 + 2 H2O H2 → CH4 + 2 H2ONow all the elements are balanced, and we didn't have to rebalance anything we had previously balanced. Balancing equations - a summaryWhen balancing equations, there are several things you should bear in mind:
States of MatterTo make a chemical equation complete, the state of matter of each substance should also be included. This indicates whether the substance is:
In this example, solid magnesium ribbon burns in oxygen gas to form solid magnesium oxide: 2 Mg (s) + O2 (g) → 2 MgO (s)
Some equations for you to balanceIn each of the following questions you will see a blank box before the symbol of each compound and element. Enter the appropriate number in each box, or leave the box blank if you think the chemical needn't have a number (i.e. the number is equivalent to '1'). When you think each equation is balanced, click on the small gray button that appears below it. |
 Back to the chemistry menu
Back to the chemistry menu