
Revision Questions: The Particle Model, PressureWherever you see an input box in the following text, please fill in the correct words or numbers (to 2 d.p. where appropriate). You might well need a calculator to do this, so here are some links to a few online calculators. They all open up in new tabs, and at least one of them should work! https://www.calculator.net/scientific-calculator.html |
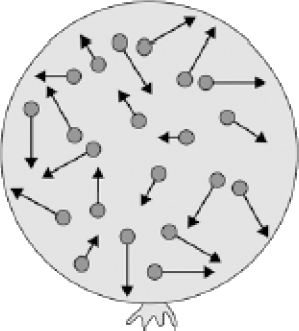 The figure on the right shows a balloon filled with helium gas.
The figure on the right shows a balloon filled with helium gas.
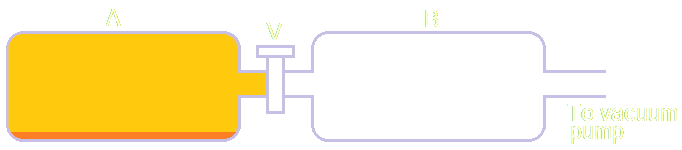 The apparatus shown on the right shows two sealed glass flasks, A and B, connected by a valve V. A small amount of liquid bromine is placed in A before V is closed and B is connected. The other connection on B is to a vacuum pump that can be used to remove all the air from B.
The apparatus shown on the right shows two sealed glass flasks, A and B, connected by a valve V. A small amount of liquid bromine is placed in A before V is closed and B is connected. The other connection on B is to a vacuum pump that can be used to remove all the air from B.